Advances in Cell Culture and Tissue Engineering
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cell Microenvironment".
Viewed by 92354Editors
Interests: cell biology; blood cells; cell culture; tissue engineering; cardiovascular medicine; biopharmaceuticals; microalgae
Special Issues, Collections and Topics in MDPI journals
Interests: 3D tissue engineering; biofabrication; in vitro models of fibrosis; angiogenesis; cell-based therapy; extracellular matrix; transglutaminases; microenvironment; macromolecular crowding; adult stem cells; spheroid culture
Special Issues, Collections and Topics in MDPI journals
Interests: engineering microvasculature; angiogenesis and revascularization; cell-based therapy; extracellular matrix; hydrogels; tissue engineering; inflammation; microenvironment; macromolecular crowding; pericytes; chronic wounds; critical limb ischemia
Topical Collection Information
Dear Colleagues,
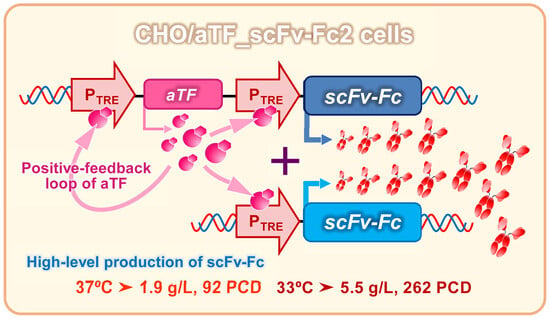
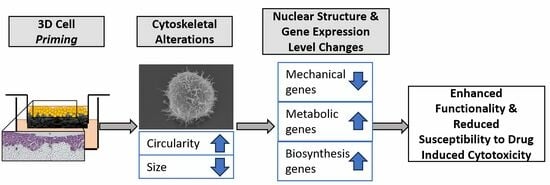
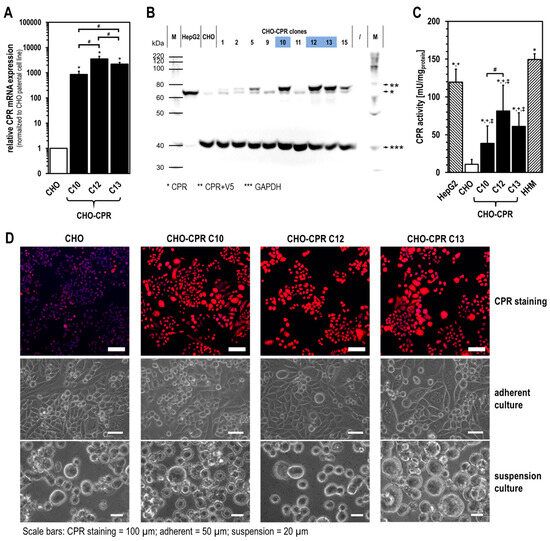
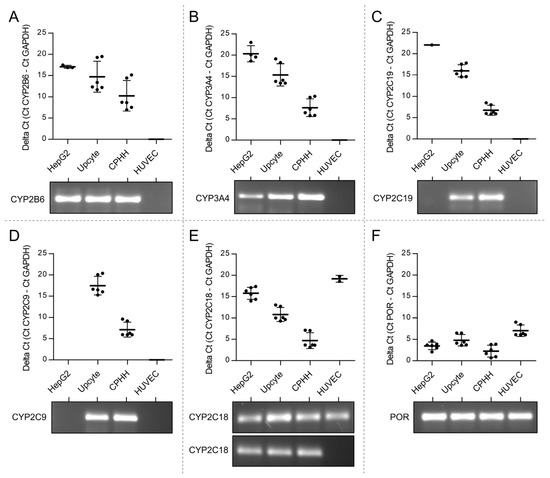
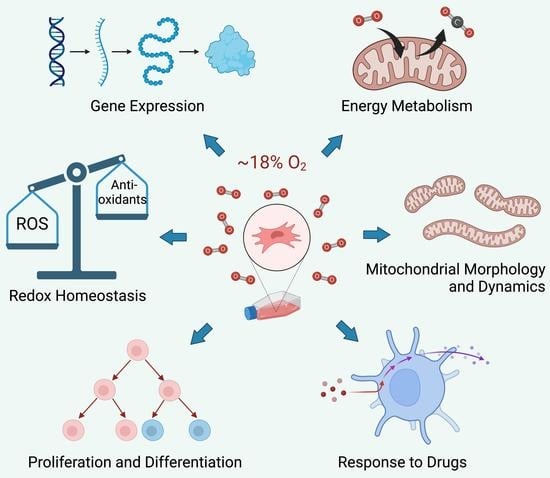
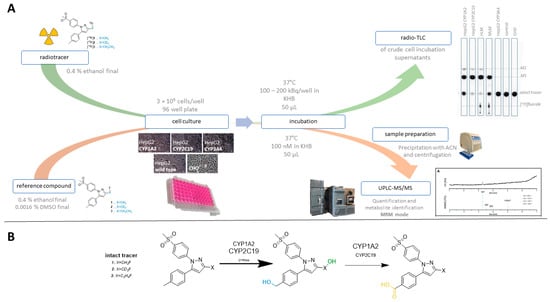
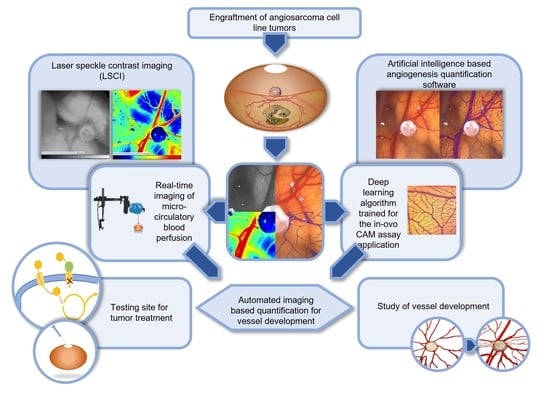
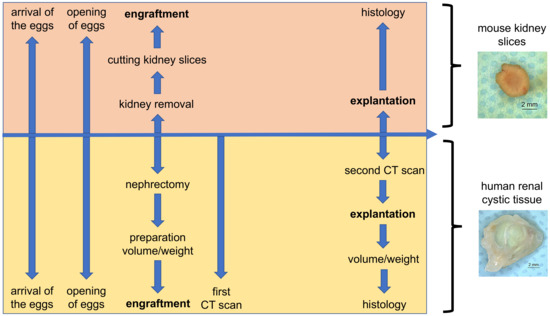
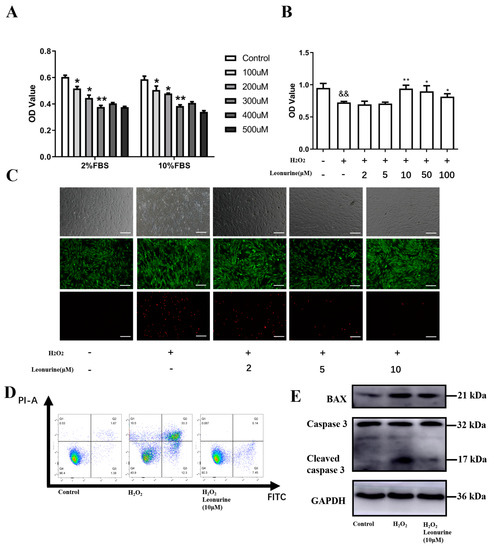
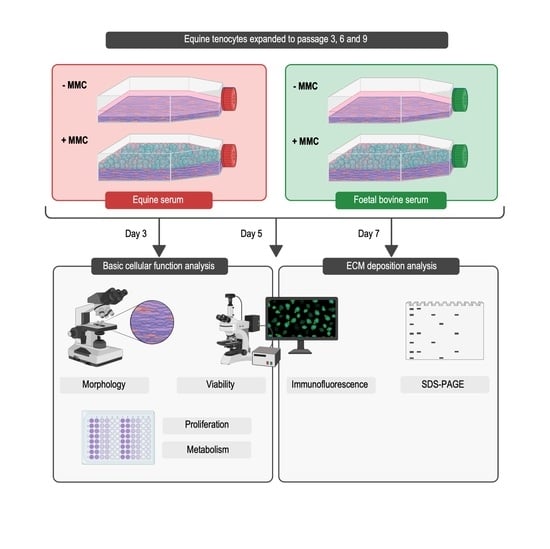
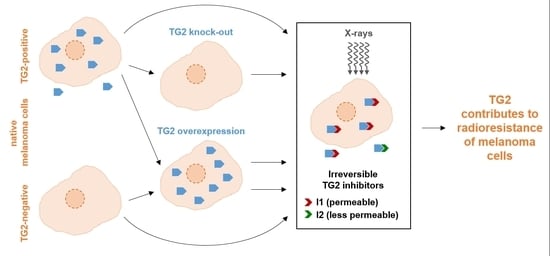
Cell culture systems are one of the most important tools in cellular and molecular biology, tissue engineering, drug discovery, and beyond. They are essential to address basic research questions, and to pave the way for therapeutic approaches. One crucial requirement of cell culture systems is their ability to resemble key aspects of their in vivo counterparts. As a result, many advances have been made toward complex 2-dimensional/3-dimensional cell cultures, ex-vivo cultures, the modeling of microenvironments, and the engineering of tissues. This Topical Collection thus aims to highlight these recent developments in cell culture systems and tissue engineering including 2-dimensional/3-dimensional cell culture systems, in studies according to (patho)physiological events, as well as toxicity studies. Such cell culture systems provide excellent model systems for the investigation of the physiological and pathophysiological responses of cells in great detail and over time, the metabolism of cells, the effects of drugs or toxic compounds on the cells, mutagenesis, and last but not least carcinogenesis. Additionally, the engineering of micro-tissues including organ-on-a-chip approaches, as well as replicating features of various microenvironments in vitro are issues of interest.
Prof. Dr. Friedrich Jung
Prof. Dr. Michael Raghunath
Prof. Dr. Anna Blocki
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- cells
- 2D/3D culture
- ex vivo culture
- tissue engineering
- microenvironment
- organ-on-a-chip